Dr Hayden Homer | IVF Doctor Brisbane
IVF is a highly effective form of Assisted Reproductive Treatment (ART) and the most advanced from of fertility treatment available. The use of IVF is increasing rapidly. It is estimated that at least 1 child in every classroom in Australia is the result of IVF. Dr Hayden Homer is a leading IVF Doctor based at his advanced IVF clinic in Brisbane.
What is IVF and what are the steps involved?
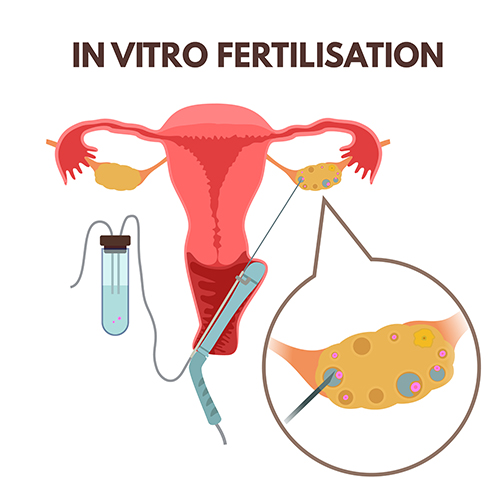
IVF is the most advanced form of fertility treatment and stands for In Vitro Fertilisation. Translated, it means fertilisation “in glass”, referring to the fact that in IVF, the two reproductive cells, sperm and egg, are brought together outside of the body. Fertilisation of the egg by the sperm produces the earliest stage of pregnancy, known as an embryo, which is then placed into the cavity of the womb.
IVF involves the following steps: (1) Ovarian stimulation to produce multiple eggs; (2) Removal of these eggs from the body via a transvaginal ultrasound-guided Egg Pickup (EPU); (3) Sperm production and preparation in the lab; (4) Bringing the eggs and sperm together in the lab to enable fertilisation to occur to produce embryos; (5) Culture of these embryos in the lab for 2-6 days, and; (6) The replacement of an embryo into the womb via Embryo Transfer (ET).

How is ovarian stimulation performed?
During a woman’s natural menstrual cycle, one egg develops within a chamber called a follicle. After the follicle has developed to an advanced stage, it ruptures to release a mature egg from the surface of the ovary, termed ovulation. Growth of the follicle occurs under the influence of the hormone, Follicle Stimulating Hormone (FSH), that is produced by a very small gland at the base of the brain called the pituitary gland.
One of the main objectives during IVF is to produce multiple eggs (not just one egg as during a natural cycle). To achieve this, FSH is administered by daily injection under the skin of the tummy, usually for around 8-14 days; this is referred to as ovarian stimulation. FSH and other IVF medications are available in easy-to-use “pen” format.

During this time follicle development is monitored using transvaginal ultrasound scanning and blood tests for measuring the levels of the hormone, oestrogen. With a good response, more than 3 follicles will grow beyond 10 mm at the rate of roughly 1-2 mm per day and since follicles produce the majority of the circulating oestrogen, oestrogen levels will also rise. Increasing levels of oestrogen are sensed by the pituitary and can cause the pituitary to release the hormone, luteinising hormone (LH), in the form of a so-called “LH surge” which triggers ovulation. During IVF, it is important to avoid such an LH surge since this would cause the eggs to be released within the body prior to their retrieval. To prevent the surge, an LH “blocker” is also used. Typically, this is used in the form of an “antagonist” medication that is introduced around the fifth day of FSH injections.
When follicles have become large enough (typically around 1.7-2 cm in diameter), this is a sign that the egg inside the follicle can be induced to “mature”. This maturation process is brought about by the “trigger” injection, which is usually human chorionic gonadotrophin (hCG) hormone. The hCG trigger has the same effect as the body’s LH surge and is given around 36 hours prior to planned egg pick-up. Since only mature eggs can be fertilised by sperm, without the trigger, there will be no mature eggs and the IVF cycle will be unsuccessful.
How is the egg pickup performed?
Following administration of the trigger, the egg pickup (EPU) needs to be performed within a narrow time frame, usually around 34-37 hours after the trigger. EPU needs to be long enough after the trigger to allow time for the eggs to mature, but not too long after, as this will allow time for the eggs to be released from the follicles within the body.
EPU is performed in an operating theatre. It can be done under general anaesthetic or using light sedation. Transvaginal ultrasound is used to visualise the follicles and to safely guide a needle through the vaginal wall directly into each large follicle within the ovary.
Using a suction pump, the fluid from each of the follicles is aspirated into a test-tube. This fluid is then inspected under a high-powered microscope by the embryology scientist to determine whether an egg has been obtained. Not every follicle produces an egg.

The EPU procedure usually lasts around 30 minutes. Following EPU, there is often some discomfort from the ovaries, which is easily managed with pain-killers. In trained hands, EPU is a very safe procedure but occasionally complications can occur. The commonest one is bleeding, which usually settles on its own.
How is sperm obtained for fertilising eggs?
In most cases, the male partner can produce sperm through ejaculation. Sperm is produced on the same day as the EPU as a fresh sperm sample gives the best fertilisation results.
If sperm cannot be produced through ejaculation, for instance, because the man has had a vasectomy, then sperm can be surgically removed from the testes (known as a TESA) or from the tube leading from the testes called the epididymis (known as a PESA). These surgical sperm retrievals are performed under either general or local anaesthesia. They involve placing a needle into the testes (for TESA) or into the epididymis (for PESA).
The third source of sperm is from frozen specimens stored in “straws”. Sperm may have been frozen by the man prior to IVF treatment or may be from a third-party donor. On the day of EPU, one or more straws of sperm is thawed for fertilising the eggs.
How is fertilisation performed?
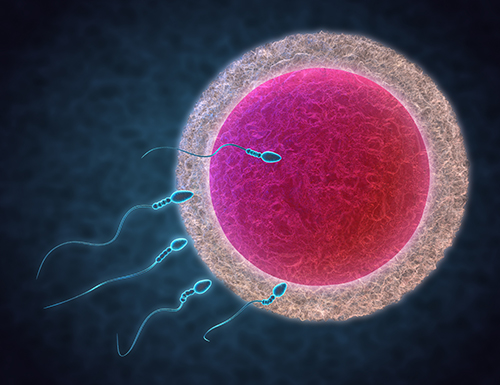
Fertilisation involves bringing sperm and eggs together in the lab, termed insemination. If semen analyses or previous IVF treatment shows that the sperm is of good quality, insemination can be performed by simply mixing a prepared volume of sperm with eggs in tiny wells of culture media within a Petri dish and allowing the sperm to fertilise the egg on its own. This is often referred to as standard IVF.
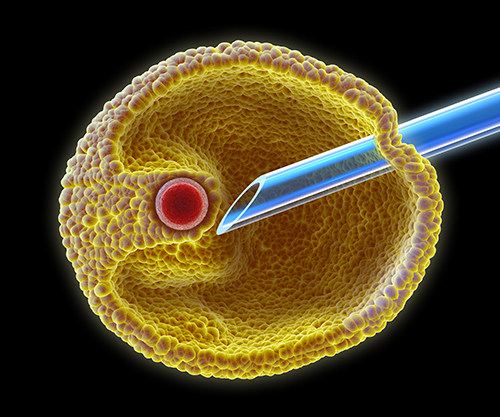
The other method for fertilising the egg is called Intra Cytoplasmic Sperm Injection or ICSI. This procedure is typically used when sperm quality is low, which reduces the chances of it being able to fertilise the egg on its own. During ICSI, a single sperm is selected and, using a high-powered microscope, the sperm is injected into the egg’s substance (known as cytoplasm) using a very fine needle that punctures the eggs outer layers.
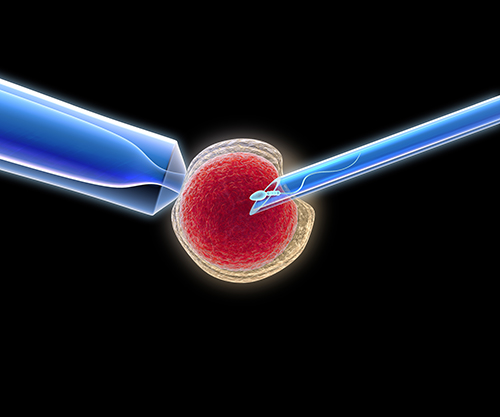
The movie below depicts mouse eggs being microinjected with a solution of RNA in Prof Homer’s research laboratory. The process is very similar to ICSI and involves stabilising the egg by applying suction through a tiny glass holding pipette (left) and introducing the sharp injection needle through the egg’s outer layers and into the egg substance (right). A pulse of pressure is then applied into the injection needle to expel its contents into the egg.
What happens to the embryo after fertilisation?
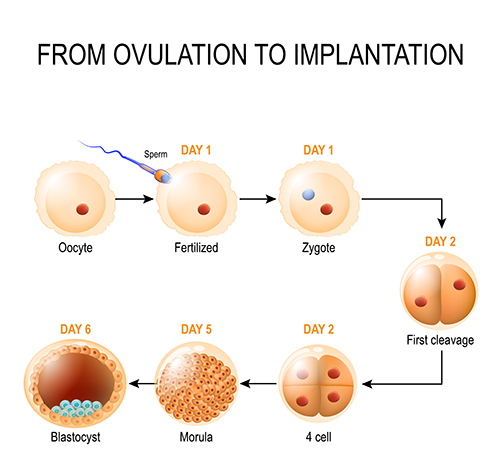
The day after bringing the sperm and eggs together, the embryologists check to see how many eggs got fertilised. They can determine this by checking for the presence of two “pronuclei” in the egg; one pronucleus comes from the sperm and the other from the egg and is proof that the sperm penetrated and fertilised the egg. Occasionally abnormal fertilisation occurs and 3 pronuclei are seen. Typically, around 70-80% of eggs become fertilised. The pronuclear stage is the earliest embryonic stage, also known as the zygote.
Embryos are then maintained in the lab within specialised media droplets under strictly controlled temperature and gassing conditions. Embryo development is monitored by the embryologists using microscopes. This very early embryo is referred to as the preimplantation embryo. Preimplantation development involves “cleavage” into smaller cells; as cell numbers increase, the size of individual embryonic cells decreases. By Days 4-5 of development, the embryo has typically developed into a morula, and by Days 5-6, it reaches the blastocyst stage.
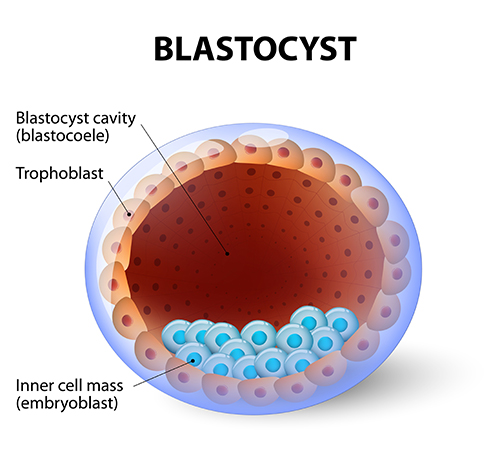
The blastocyst has a small fluid-filled cavity (the “blastocoele”) and at this stage, the embryo now has two distinct cell-types, the outer “shell” or trophectoderm and an inner cell mass. The trophectoderm will form the placenta and the inner cell mass will form the fetus.
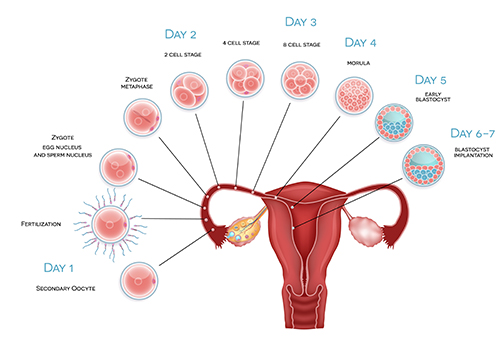
During natural pregnancy, eggs become fertilised at the outer end of the Fallopian tube and subsequent steps of embryo development occur as the embryo makes its way along the tube towards the cavity of the womb. The embryo is usually at the blastocyst stage by the time it reaches the womb cavity and begins implantation. It is for this reason that blastocyst stage embryos often produce higher success rates during IVF than earlier cleavage-stage embryos. Professor Homer wrote the chapter on Fertilisation in one of the world’s most popular textbooks of Gynaecology (Gynaecology 4th Edition; Chapter 19).
When is embryo transfer performed?
Embryos are transferred at either the cleavage-stage (Day 2 or 3) or the blastocyst-stage (Day 5 or 6). The particular stage at which the embryo is transferred depends on a number of factors. Two of the main determinants are how many embryos are available and what their quality looks like. Because the lab is a stressful environment for the embryo, weaker embryos are transferred early rather than late, that is, by Day 2/3 rather than Day 5/6. The reason for this is that weak embryos that still have a chance of making a baby within the womb might succumb if left too long in laboratory conditions.
Is the womb lining important and how is the lining prepared for receiving the embryo?
The womb lining is very important for IVF success because the embryo needs to embed in the lining by a process called implantation for pregnancy to occur. Implantation will only be successful if the embryo is of good quality and the lining is suitably prepared (or receptive) for the embryo. Professor Homer wrote the chapter on Implantation in one of the world’s most popular textbooks of Gynaecology (Gynaecology 4th Edition; Chapter 19).
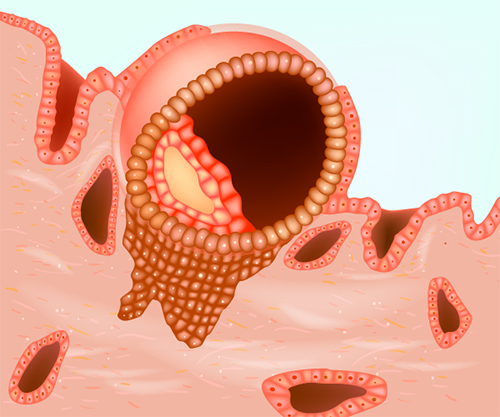
During ovarian stimulation, in addition to monitoring follicle development, it is therefore very important to evaluate the womb lining for its appearance as well as thickness. A lining that is developing well during simulation will show three clear “lines” (or a trilaminar appearance) due to the effect of oestrogen and will usually increase in thickness to around 7mm or more.
To make the womb lining receptive to the embryo requires the hormone, progesterone. The follicles that have had their eggs removed at egg pickup will produce some progesterone but this will not be enough. Following EPU, it is therefore very important to start using progesterone medication. Progesterone is most often inserted into the vagina in the form of a gel or pessary. Depending on the particular product, it may need to be inserted once, twice or three times per day. Occasionally, progesterone is given in the form of injections into the muscle. It is important that progesterone is continued at least until the time of pregnancy test.
Occasionally, pregnancy fails to occur despite the repeated transfer of very good quality embryos; this is called recurrent implantation failure. There are a number of possible reasons that have been suggested to account for recurrent implantation failure, for instance, an imbalance in immune factors, a tendency to form clots etc. A recently developed test called the Endometrial Receptivity Assay (ERA) may be helpful in this situation. ERA is used to analyse a sample of the lining that is taken at a stage in the cycle when implantation is expected to occur. It measures the expression levels of a panel of genes that are important for receptivity and implantation and can give advice on the most appropriate time for performing the embryo transfer.
How is embryo transfer performed and should ultrasound scanning be used to guide transfer?
Embryo transfer is performed in an operating theatre adjacent to the embryology lab where the embryos are being cultured. The procedure is similar in some ways to having a Pap smear performed and no anaesthetic is required. Legs are placed in supports and the neck of the womb is exposed using a speculum, identical to having a Pap smear. After cleaning the neck of the womb, a small tube is placed through the neck of the womb and into the lower aspect of the womb cavity. The embryologists then load the embryo into a second, even finer, tube using a microscope. This very fine tube is then threaded through the outer tube that was previously positioned within the womb, and when it is at precisely the right spot in the cavity, the embryo is released from the tube into the womb cavity.
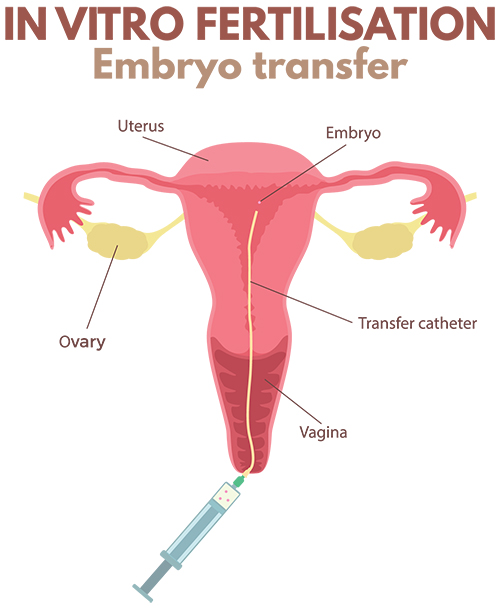
It is strongly recommended that ultrasound scanning on the tummy be used during embryo transfer since there is very clear evidence that using ultrasound to guide the placement of the tube in the cavity prior to embryo release produces the best pregnancy success rates. To facilitate scanning on the tummy, a full bladder is required. The specialist scans on the tummy at the same time as the transfer is being performed to ensure that the embryo is released into the correct position within the womb.
What happens after embryo transfer?
On the day of embryo transfer, it is advisable to avoid strenuous activity. Following embryo transfer, it is very important to continue using progesterone. Pregnancy test will be performed with a blood test around 10-14 days after embryo transfer depending on the stage at which the embryo was transferred. Many specialists advise continuing progesterone for 8-10 weeks if the pregnancy test is positive. Using progesterone after positive pregnancy test will incur an additional cost since additional progesterone beyond pregnancy test is not covered by PBS.
What happens if I have extra embryos left over after my embryo transfer?
IVF cycles often produce more than one embryo. As a result, there may be unused embryos after embryo transfer. If these embryos are of suitable quality, they can be frozen (or cryopreserved). In some cases, for instance, when the risk of OHSS is high, all embryos from the cycle will be frozen to prevent OHSS from developing. Embryo freezing allows you to have more than one pregnancy from a single IVF stimulation cycle.
Frozen embryos are thawed and replaced in Frozen Embryo transfer (FET) cycles. Following thaw, more than 90% of embryos return to a very similar state to what they were before being frozen. A small proportion of embryos do not survive thawing. FET cycles can be completely natural in women who ovulate regularly. In natural FET cycles, the body’s own hormone production is used to prepare the lining of the womb to receive the embryo and no additional medication is needed. Alternatively, FET cycles can be medicated, and involve the use of oestrogen and progesterone medications to prepare the lining.
What are my chances of success with IVF and why are they so dependent on the age of the female partner?
The rate-limiting factor for IVF success is the quality of the embryo. Embryo quality in turn is almost entirely dependent upon egg quality. All the eggs a woman will ever have are those that she was born with; no new eggs are produced after birth. Consequently, as a woman gets older, so too do her eggs. As eggs age, their capacity to make good embryos and to support pregnancy also declines. By the start of the thirties, this egg-decline begins to have a marked impact on embryo- and pregnancy-potential and becomes particularly pronounced after the mid-thirties. It is for these reasons that IVF success is directly related to female age and why IVF success rates are typically reported with reference to female age.
Because of this impact of female age, the highest IVF success rates occur for women under 35 years of age. Success rates become markedly reduced after the age of 37 years and especially after 40 years of age. Because of this ageing effect, the success rate for someone in their late thirties is roughly half that of someone in their early thirties.
The success of an IVF cycle is also strongly influenced by the quality of the embryology laboratory and importantly, the expertise of the treating clinician.
Professor Homer is an internationally leading expert in egg quality and the effects of ageing. His lab is actively researching novel treatments for reversing poor egg quality. Click here to read his recent paper on the effect of ageing on egg quality published in the world’s top reproduction journal.
What about add-ons in IVF?
Add-ons are “extras” used in IVF because it is hoped they might improve success rates. Add-ons can be considered as belonging to two groups; those used during the making or selection of embryos and those that aim to improve the interaction between the embryo and the womb lining (or implantation). Add-ons from the former group include Physiological ICSI (PICSI, a modified form of ICSI where sperm undergo a selection test prior to ICSI), Preimplantation Genetic Testing for Aneuploidy (PGT-A) and Timelapse Embryo Monitoring. Add-ons aimed at improving implantation include Natural Killer (NK) Cell testing, Endometrial Scratching and Embryo Glue.
The reality about add-ons is that they are expensive and evidence is very unclear whether they can definitively improve pregnancy chances. Indeed, in some cases, like PICSI and endometrial scratching, there is evidence from recent large clinical trials that they don’t improve outcomes. More worrying, is that recent data suggest that PGT-A could actually worsen outcomes for some patients. Before including an add-on, there should be careful discussion about why it is being used and how it will benefit your treatment.
More information on PGT-A and NK cell testing is available in two of Prof Homer’s recent papers.
Are there any risks associated with IVF?
IVF in expert hands is a safe treatment. The main risks are associated with ovarian stimulation, the egg pickup procedure and with features of any resulting pregnancies.
In some women, ovarian stimulation produces large numbers of follicles (more than 15-20) and very high oestrogen levels (greater than 15,000 pmol/L). This is known as ovarian hyperstimulation syndrome (OHSS). OHSS can be of varying degrees of severity. Typically, OHSS is associated with swelling of the tummy due to the ovaries becoming enlarged. OHSS can also cause blood vessels to become “leaky”. Fluid leaking into the tummy will worsen abdominal distension and may cause changes in bowel activity (diarrhoea or constipation) as well as nausea and vomiting. In severe cases, fluid can accumulate in the lungs and clots can form in blood vessels. The risk of the severe form of OHSS occurring is around 1%, but there are now very effective strategies for preventing severe OHSS altogether. Women who are slim, young and have polycystic ovaries are the ones most at risk for developing OHSS.
The risks associated with the egg pickup are bleeding, infection and inadvertently puncturing a nearby structure such as bowel. The risk of damaging another structure like bowel during egg pickup is extremely low, less than 1%.
One important pregnancy-related risk linked with IVF is the production of twin and triplet pregnancies if more than one embryo was transferred. Such pregnancies are riskier than those with only one baby (singletons) since they have an increased chance of very premature delivery that can sometimes result in developmental problems for some children. In Australia, over 80% of all embryo transfers involve only one embryo so this keeps the rates of IVF twins and triplets low. Another important pregnancy-related risk that occurs during IVF is ectopic pregnancy, which is a pregnancy located in an abnormal position outside of the womb, most often in one of the Fallopian tubes. Ectopic pregnancies are a medical emergency because they may rupture and cause severe internal bleeding. Treating ectopic pregnancies often involves surgery to remove the affected tube.
Who should I see to undertake my IVF?
IVF is a highly specialised and very expensive treatment. In Queensland, IVF is not available anywhere in the public sector so a trainee doctor will require separate training to gain experience and expertise in IVF. A doctor’s credentials provide guidance on whether that doctor has undertaken the additional training required for properly undertaking IVF. In Australia, these credentials are known as CREI, which stands for Certificate in Reproductive Endocrinology and Infertility. CREI is conferred by the Royal Australian & New Zealand College of Obstetricians & Gynaecologists (RANZCOG) and is proof that a doctor has undergone an extensive period (3 years and more) of sub-specialty training in IVF and all other aspects related to infertility management.
Prof Homer is one of only four CREI sub-specialists in Queensland. He is the only one who has also received REI sub-specialty accreditation from the UK’s Royal College of O&G. Prior to relocating to Australia, Prof Homer worked and trained at the Centre for Reproductive & Genetic Health in London, the top IVF clinic in the UK. Added to this, Hayden has a PhD in fertility research into egg quality. He continues to undertake extensive research and directs Queensland’s first and only research lab dedicated to studying egg quality and developing new fertility treatments. Prof Homer therefore has a unique breadth of knowledge, experience and expertise required for maximising IVF success rates. Although he is a fully qualified Obstetrician with both the Australian and the UK Royal Colleges of O&G, Prof Homer has chosen to stop Obstetric practice so that he can commit 100% of his attention to meticulously managing his patients’ IVF cycles to ensure they have the best chance of success.